Shop
UV Smart D25
Certifications
CE Certificate
The D25 V2 received CE certification in October 2023 (MDR Class IIa).
Health Canada
The UV Smart D25 is registered with Health Canada as a Class II medical device (Licence No.: 108693)
Standards
The product complies with NEN-EN 61010-1 (Safety requirements for electrical equipment for measurement, control, and laboratory use – Part 1: General requirements).
This certificate indicates that the D25 complies with the general safety requirements for its intended purpose.
The EN 14885 standard is used as a reference for the D25 (Chemical disinfectants and antiseptics - Application of European Standards for chemical disinfectants and antiseptics). This certification means that the equipment achieves the same level of disinfection as wipes and liquids (as there is currently no guideline for UV-C).
UV Smart Technologies B.V. has held the NEN-EN-ISO 13485 certificate since May 2020. This certification is the international standard for manufacturers, suppliers and distributors of medical devices to show that they comply with the legal requirements for medical devices.
Patent Protection
Impelux™ is a patented technology that uses UV technology for guaranteed disinfection without damaging equipment and with validated and continuous effectiveness.
Technical Specifications
For disinfection of:
Non-critical medical instruments and devices
Dimensions:
- Width: 577 mm
- Depth: 488 mm
- Height: 295 mm
Disinfection Chamber:
- Width: 378 mm
- Depth: 224 mm
- Height: 150 mm
Power cable:
- Length: 2 m
Disinfection cycle:
- 25 seconds
Effective against:
- Viruses
- Bacteria
- Spores
- Yeast
- Fungi
- Mycobacteria
- Tuberculin
Clinical Evidence
The disinfection efficiency of the UV Smart D25 has been proven and supported by numerous extensive studies conducted in clinical settings at academic medical centres (University Medical Centre Groningen, University Medical Centre Amsterdam and Radboud University Medical Centre in the Netherlands) and laboratories (Eurofins and Streeklab Haarlem, the Netherlands). These studies have proven that the UV Smart D25 is very effective on the full spectrum of micro-organisms.
The D25 achieves at least a log-4 reduction in microbial counts in 25 seconds on the hard, non-porous surfaces of non-invasive medical devices. The UV Smart D25 was also able to achieve significant log reduction of methicillin-resistant Staphylococcus aureus, van-comycin-resistant enterococci (VRE), the family of Corona viruses, Norovirus and Escherichia coli.
*These studies and research are available upon request from info@biomiq.health.
Frequently Asked Questions
What products can I disinfect in the D25?
The D25 is designed for fast and effective disinfection of non-invasive medical devices, electronics and other small equipment in hospitals, health centres, nursing homes and laboratories. For example: infusion pumps, thermometers, stethoscopes, telephones, reflex hammers, badges, blood pressure pumps and much more. For tailor-made advice and additional information, please get in touch with our team.
Is the D25 also mobile?
Yes, the D25 can be delivered on a cart. This allows the D25 to be moved easily from department to department. This is an extra option of UV Smart. Attachment sold separately.
Can the data of the D25 be read out?
Yes, the data of the D25 can be read out. This can be done via a USB port at the back of the device. Each disinfection cycle (success and failure) can be viewed with all data, including time, duration, light dose, etc.
Does the D25 device demonstrate/confirm the success of a disinfection cycle?
The D25 contains optical sensors that, during each disinfection cycle, verify whether sufficient doses of UV-C light have been released for successful disinfection of the item. When the light dose approaches the threshold, the device indicates that maintenance is required. If no maintenance is performed and the dose drops below the threshold, the cabinet switches itself off. This is how the D25 prevents false reliability.
What accessories are available for the D25?
In addition to the Cart, a specially designed holder is available for the convenient storage of disinfectants or other items along with the device. Also available are disinfection cycle validation stickers—a tool to visually verify whether the UV emission was sufficient in a given disinfection cycle. A hard-shell case is another available accessory, with which the D25 can be transported in a safe, reliable manner to its destination without concern of damage.
Can I test the D25 in my facility?
To see if the D25 is a good fit in your department (and we're confident it will be!), we offer a two (2) week trial placement. This is a great opportunity to get familiar with what Impelux™ and UV-C technologies can do for your organization in the fight against healthcare-associated infections.
Can I test the D45 in my hospital/clinic?
To see if the D45 fits in your workflow, you can request a 2-week trial placement. This will give you an idea of what the product can do for your organization in the fight against healthcare-associated infections.
Downloads
In search of something else? Contact us to request additional resources about this product.
Product Description
Automated. Fast. No Consumables.
The UV Smart D25 is a UV-C based high level disinfection device designed for fast and highly effective disinfection of non-critical and semi-critical medical devices, as well as electronics and other small equipment in hospitals, health centres, nursing homes and laboratories.
The D25 is effective against the full spectrum of microorganisms, and features Impelux™ technology—a patented technology that assures that the energy applied is as high as possible while hitting the surface of the medical object, resulting in a reliable, and very short, disinfection cycle.
Product Description
Automated. Fast. No Consumables.
The UV Smart D25 is a UV-C based high level disinfection device designed for fast and highly effective disinfection of non-critical and semi-critical medical devices, as well as electronics and other small equipment in hospitals, health centres, nursing homes and laboratories.
The D25 is effective against the full spectrum of microorganisms, and features Impelux™ technology—a patented technology that assures that the energy applied is as high as possible while hitting the surface of the medical object, resulting in a reliable, and very short, disinfection cycle.
Certifications
CE Certificate
The D25 V2 received CE certification in October 2023 (MDR Class IIa).
Health Canada
The UV Smart D25 is registered with Health Canada as a Class II medical device (Licence No.: 108693)
Standards
The product complies with NEN-EN 61010-1 (Safety requirements for electrical equipment for measurement, control, and laboratory use – Part 1: General requirements).
This certificate indicates that the D25 complies with the general safety requirements for its intended purpose.
The EN 14885 standard is used as a reference for the D25 (Chemical disinfectants and antiseptics - Application of European Standards for chemical disinfectants and antiseptics). This certification means that the equipment achieves the same level of disinfection as wipes and liquids (as there is currently no guideline for UV-C).
UV Smart Technologies B.V. has held the NEN-EN-ISO 13485 certificate since May 2020. This certification is the international standard for manufacturers, suppliers and distributors of medical devices to show that they comply with the legal requirements for medical devices.
Patent Protection
Impelux™ is a patented technology that uses UV technology for guaranteed disinfection without damaging equipment and with validated and continuous effectiveness.
Technical Specifications
For disinfection of:
Non-critical medical instruments and devices
Dimensions:
- Width: 577 mm
- Depth: 488 mm
- Height: 295 mm
Disinfection Chamber:
- Width: 378 mm
- Depth: 224 mm
- Height: 150 mm
Power cable:
- Length: 2 m
Disinfection cycle:
- 25 seconds
Effective against:
- Viruses
- Bacteria
- Spores
- Yeast
- Fungi
- Mycobacteria
- Tuberculin
Clinical Evidence
The disinfection efficiency of the UV Smart D25 has been proven and supported by numerous extensive studies conducted in clinical settings at academic medical centres (University Medical Centre Groningen, University Medical Centre Amsterdam and Radboud University Medical Centre in the Netherlands) and laboratories (Eurofins and Streeklab Haarlem, the Netherlands). These studies have proven that the UV Smart D25 is very effective on the full spectrum of micro-organisms.
The D25 achieves at least a log-4 reduction in microbial counts in 25 seconds on the hard, non-porous surfaces of non-invasive medical devices. The UV Smart D25 was also able to achieve significant log reduction of methicillin-resistant Staphylococcus aureus, van-comycin-resistant enterococci (VRE), the family of Corona viruses, Norovirus and Escherichia coli.
*These studies and research are available upon request from info@biomiq.health.
Frequently Asked Questions
What products can I disinfect in the D25?
The D25 is designed for fast and effective disinfection of non-invasive medical devices, electronics and other small equipment in hospitals, health centres, nursing homes and laboratories. For example: infusion pumps, thermometers, stethoscopes, telephones, reflex hammers, badges, blood pressure pumps and much more. For tailor-made advice and additional information, please get in touch with our team.
Is the D25 also mobile?
Yes, the D25 can be delivered on a cart. This allows the D25 to be moved easily from department to department. This is an extra option of UV Smart. Attachment sold separately.
Can the data of the D25 be read out?
Yes, the data of the D25 can be read out. This can be done via a USB port at the back of the device. Each disinfection cycle (success and failure) can be viewed with all data, including time, duration, light dose, etc.
Does the D25 device demonstrate/confirm the success of a disinfection cycle?
The D25 contains optical sensors that, during each disinfection cycle, verify whether sufficient doses of UV-C light have been released for successful disinfection of the item. When the light dose approaches the threshold, the device indicates that maintenance is required. If no maintenance is performed and the dose drops below the threshold, the cabinet switches itself off. This is how the D25 prevents false reliability.
What accessories are available for the D25?
In addition to the Cart, a specially designed holder is available for the convenient storage of disinfectants or other items along with the device. Also available are disinfection cycle validation stickers—a tool to visually verify whether the UV emission was sufficient in a given disinfection cycle. A hard-shell case is another available accessory, with which the D25 can be transported in a safe, reliable manner to its destination without concern of damage.
Can I test the D25 in my facility?
To see if the D25 is a good fit in your department (and we're confident it will be!), we offer a two (2) week trial placement. This is a great opportunity to get familiar with what Impelux™ and UV-C technologies can do for your organization in the fight against healthcare-associated infections.
Can I test the D45 in my hospital/clinic?
To see if the D45 fits in your workflow, you can request a 2-week trial placement. This will give you an idea of what the product can do for your organization in the fight against healthcare-associated infections.
Downloads
In search of something else? Contact us to request additional resources about this product.
True disinfection starts here.

Connect with us 1-1 to learn more.
No matter where you’re located, we would love to connect with you and learn about your facility’s needs in device disinfection.
Use the links below to schedule a meeting time convenient for you.
EASTERN CANADA
Book a TimeCENTRAL CANADA
Book a TimeWESTERN CANADA
Book a TimeNORTHERN CANADA
Book a TimeAdvanced HLD for Hospitals, Health Centres, LTC and Labs
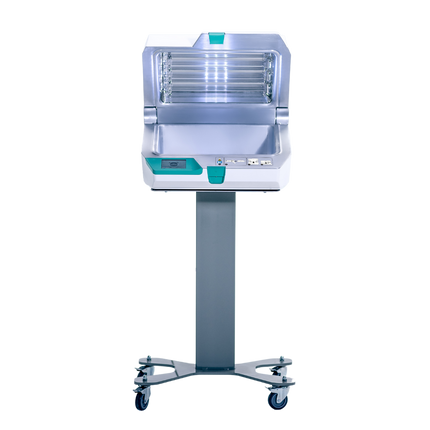
For high-energy and fast disinfection. A consistent disinfection cycle in only 25 seconds.
Validated and proven by clinical research. Medically certified UV-based device (CE class IIa).
Achieves a minimum log-4 reduction on the full spectrum of infection-causing micro-organisms.
Disinfection without chemicals or water use. UV technology lamps with low energy consumption.
25 seconds is all it takes.
Experience the UV-C DifferenceContact us today for your free two (2) week trial placement of a D25 device in your facility, risk free.
4 Easy Steps to Complete Device Disinfection
Here's how to use the UV Smart D25.

Step 1: Preparation
Remove visible dirt or debris from the device you're intending to disinfect.

Step 2: Start the Process
Gently place the item in the D25 device and close the lid.

Step 3: Disinfect Hands
The cycle will take just 25 seconds. Disinfect your hands while waiting.

Step 4: Cycle Complete!
The lid will open automatically when the cycle is finished. Retrieve your item from D25.
The Green Standard
Cleaner. Greener. Leaner.The Green Standard promotes sustainability in healthcare infection prevention by providing a framework for incorporating UV-C disinfection devices efficiently into healthcare processes while prioritizing patient safety, sustainability and workflow efficiency.